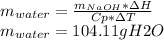Answer:
104.11 of solution could be heated till boiling at 1atm
Step-by-step explanation:
For calculating this value, we are going to calculate it according to water whose heat capacity is 4.184 J/gC. We are going to use the value of entalphy of solution of the NaOH (-44.51kJ/mol in water at 25 celsius).
So, we have a heat balance
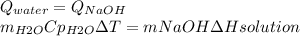
Now, we know we have to calculate the mass of water and we know that water was initially at 25ºC, so we are going to take it into 100ºC. We also know that the heat of solution is given at kJ mol, so we have to do a transformation of units so we have the correct answer. We are going to change kJ into J and moles into grams
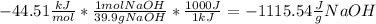
Now, changing the values into the heat balance we obtain