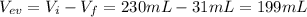Answer:
The volume of water evaporated is 199mL
Step-by-step explanation:
Concentration is calculated with the following formula
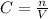
where n is the number of moles of solute and V is the volume of the solution (in this case is the same as the solvent volume) in liters.
So we isolate the variable n to know the amount of moles, using the volume given in liters
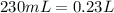
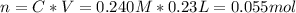
Now, we isolate the variable V to know the new volume with the new concentration given.
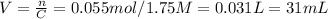
Finally, the volume of water evaporated is the difference between initial and final volume.