Step-by-step explanation:
Given that,
Radius of divalent cation,
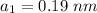
Radius of monovalent anion,
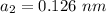
Charge for divalent cation,

Charge for monovalent cation,
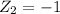
The sum of radii of both cations and anion is,
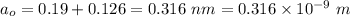
(a) The force of attraction between two ions is given by :
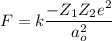
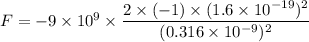
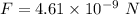
(b) Let F' is the force of repulsion at this same distance such that,
F + F' = 0
So,
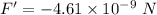
Hence, this is the required solution.