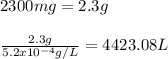Answer:
4423.08 L
Step-by-step explanation:
First we need to know the concentration of sodium per litre (as the density is 1 g/L, 1 g of water is the same that 1 mL of water) of softened water by dividing the percentage in 100:
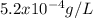
With the value of concentration, we just need to to divide the recommended value of the FDA, that is 2300 milligrams per day, into the concentration that we have, therefore: