Answer: 0.4 moles of glucose are produced by the reaction of 2.40 moles of water.
Step-by-step explanation:
Photosynthesis is a phenomenon in which green plants containing chlorophyll use sunlight as a source of energy to convert carbon dioxide and water to form glucose and oxygen.
The balanced chemical equation is:
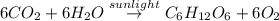
According to stoichiometry:
6 moles of water produces = 1 mole of glucose
2.40 moles of water produces =
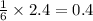 moles of glucose
moles of glucose
Thus 0.4 moles of glucose are produced by the reaction of 2.40 moles of water.