Answer:
a. Gas particles have most of their mass concentrated in the nucleus of the atom
Step-by-step explanation:
The main postulates of the molecular kinetic theory are:
A gas consists of a set of small particles that move with rectilinear motion and obey Newton's laws (this means that this theory doesn't divide the atom in a nucleus and electrons, this is why a. is not correct)
The molecules of a gas do not occupy volume (this is assumed because between the molecules of a gas is a lot of space).
Collisions between the molecules are perfectly elastic (this means that energy is not gained or lost during the crash).
There're no forces of attraction or repulsion between the molecules (because each molecule is "far" of the others).
The average kinetic energy of a molecule is
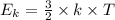 (where T is the absolute temperature and k the Boltzmann constant. This means that kinetic energy is directly proportional to temperature).
(where T is the absolute temperature and k the Boltzmann constant. This means that kinetic energy is directly proportional to temperature).