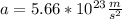Answer:
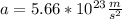
Step-by-step explanation:
In this case we will use the Bohr Atomic model.
We have that:
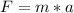
We can calculate the centripetal force using the coulomb formula that states:
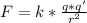
Where K=
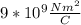
and r is the distance.
Now we can say:
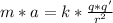
The mass of the electron is =
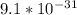 Kg
Kg
The charge magnitud of an electron and proton are=
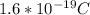
Substituting what we have:
![[tex]a=(9*10^(9)*(1.6*10^(-19) )*(2(1.6*10^(-19) )))/(9.1*10^(-31)*(2.99*10^(-11))^2 )](https://img.qammunity.org/2020/formulas/physics/college/vlj3su1kwg2k57x1ejkoovytwkl8uwz8zx.png) [/tex]
[/tex]
so: