Answer: Option (c) is the correct answer.
Step-by-step explanation:
Entropy is defined as the degree of randomness that is present within the particles of a substance.
As
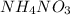 is ionic in nature. Hence, when it is added to water then it will readily dissociate into ammonium ions (
is ionic in nature. Hence, when it is added to water then it will readily dissociate into ammonium ions (
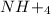 ) and nitrate ions (
) and nitrate ions (
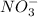 ).
).
Therefore, it means that ions of ammonium nitrate will be free to move from one place to another. Hence, there will occur an increase in entropy.
Thus, we can conclude that ammonium nitrate (
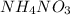 ) dissolve readily in water even though the dissolution process is endothermic by 26.4 kJ/mol because the overall entropy of the system increases upon dissolution of this strong electrolyte.
) dissolve readily in water even though the dissolution process is endothermic by 26.4 kJ/mol because the overall entropy of the system increases upon dissolution of this strong electrolyte.