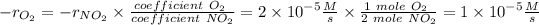Answer:
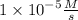
Step-by-step explanation:
The stoichiometry for this reaction is
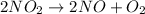
The rate for this reaction can be written as
![-r_(NO_2)=-(d\left[NO_2\right])/(dt)=((0.01-0.008)M)/(100s)=2*{10}^(-5)(M)/(s)](https://img.qammunity.org/2020/formulas/chemistry/college/1rd9bzciippi1smcn2bvu0cvrw9keza312.png)
This rate of disappearence of
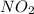 can be realated to the rate of appearence of
can be realated to the rate of appearence of
 as follows (the coefficients of each compound are defined by the stoichiometry of the reaction)
as follows (the coefficients of each compound are defined by the stoichiometry of the reaction)