Answer:
4.81%
Step-by-step explanation:
Experimental error (also known as percent error) is the positive difference between the calculated value and the actual value divided by the actual value.
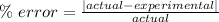
The actual density of aluminum is 2.70 g/cm³. So the experimental error is:
%error = |2.70 − 2.83| / 2.70
%errror = 0.13 / 2.70
%error = 0.0481
%error = 4.81%