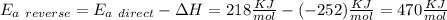Answer:
Step-by-step explanation:
The activation energy represents the energy barrier that reagents must pass to transform into products (or products to transform into reagents in a reverse reaction)
For any reaction, the change in enthalpy is related to the activation energy by the equation
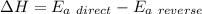
So, the activation energy for the reverse reaction is