Step-by-step explanation:
The nucleus of an atom can be modeled as several protons and neutrons closely packed together.
Mass of the particle,
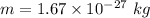
Radius of the particle,
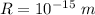
(a) The density of the nucleus of an atom is given by mass per unit area of the particle. Mathematically, it is given by :
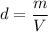 , V is the volume of the particle
, V is the volume of the particle
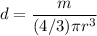
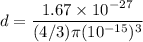
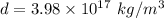
So, the density of the nucleus of an atom is
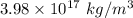 .
.
(b) Density of iron,
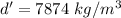
Taking ratio of the density of nucleus of an atom and the density of iron as :
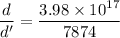
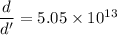
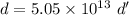
So, the density of the nucleus of an atom is
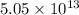 times greater than the density of iron. Hence, this is the required solution.
times greater than the density of iron. Hence, this is the required solution.