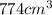Answer:
volume of water in the kettle, V =
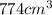
Given:
Power output of burner, P = 2.0 kW = 2000 W
Mass of kettle, m = 810 g = 0.81 kg
Temperature of water in the kettle, T =
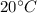
Time taken by water to boil, t = 2.4 min = 144 s
Temperaturre at boiling, T' =
Solution:
Now, we know that:
Iron's specific heat capacity,
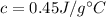
Water's specific heat capacity,
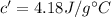
Now,
Total heat, q = Pt
q =
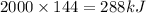
Now,
q = (mc +m'c')(T' - T)
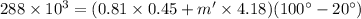
Solving the above eqn m', we get:
m' = 774 g
Now, the volume of water in the kettle, V:
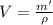
where
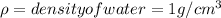
Now,
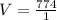
Volume, V =