Step-by-step explanation:
A chemical reaction equation that contains same number of atoms on both reactant and product side is known as a balanced chemical reaction equation.
For example,
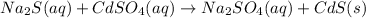
Here, number of reactant molecules are as follows.
Na = 2
S = 2
Cd = 1
O = 4
Number of product molecules are as follows.
Na = 2
S = 2
Cd = 1
O = 4
As there are already equal number of atoms on both reactant and product side. Hence, the reaction equation is balanced.
Also, in this reaction both
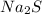 and
and
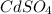 are present as aqueous solution. And, CdS is the precipitate that is formed as it is the insoluble solid that forms.
are present as aqueous solution. And, CdS is the precipitate that is formed as it is the insoluble solid that forms.
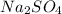 is present in aqueous state.
is present in aqueous state.