Answer: The molar mass of the unknown gas is 59.8 g/mol
Step-by-step explanation:
According to the ideal gas equation:-

P= Pressure of the gas = 706 mmHg = 0.93 atm (760mmHg=1atm)
V= Volume of the gas = 1.64 L
T= Temperature of the gas = 59°C=(59+273)K=332 K (0°C = 273 K)
R= Value of gas constant = 0.0821 Latm\K mol
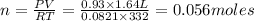
To calculate the moles, we use the equation:
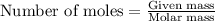
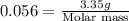
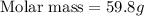
Thus the molar mass of the unknown gas is 59.8 g/mol