Answer:
The rate is
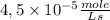
Step-by-step explanation:
Stoichiometry
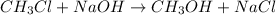
Kinetics
![-r_(A)=k * [CH_(3)Cl] * [NaOH]](https://img.qammunity.org/2020/formulas/chemistry/college/fyn7x79ndaree8t9cmcf9z18senu0swtfq.png)
The rate constant K can be calculated by replacing with the initial data
![1 * 10^(-4)(mole)/(Ls)=k * [0,2M] * [1,0M] =5 * 10^(-4)(L)/(mole s)](https://img.qammunity.org/2020/formulas/chemistry/college/d27ja5ixtwrfxtlqg3s9nb4hegkor39zxy.png)
Taking as a base of calculus 1L, when half of the
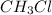 is consumed the mixture is composed by
is consumed the mixture is composed by
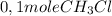 (half is consumed)
(half is consumed)
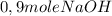 (by stoicheometry)
(by stoicheometry)
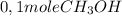
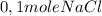
Then, the rate is
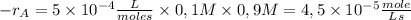
The reaction rate decreases because there’s a smaller concentration of reactives.