Answer:
0.375 grams are needed to make 25 mL solution.
Step-by-step explanation:
Mass of
 cuprous nitrate required to make 1 l of solution = 15 g.
cuprous nitrate required to make 1 l of solution = 15 g.
1 L = 1000 mL
Mass of
 cuprous nitrate required to make 1000 mL of solution = 15 g
cuprous nitrate required to make 1000 mL of solution = 15 g
Mass of
 cuprous nitrate required to make 1 mL of solution:
cuprous nitrate required to make 1 mL of solution:
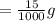
Mass of
 cuprous nitrate required to make 25 mL of solution:
cuprous nitrate required to make 25 mL of solution:
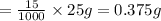
0.375 grams are needed to make 25 mL solution.