Answer:
10
Step-by-step explanation:
The atomic number of Nickel is 28: this means that a neutral atom of nickel has 28 electrons.
The electronic configuration of nickel is:
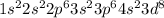
abbreviated, this is written as
![[Ar]4 s^2 3d^8](https://img.qammunity.org/2020/formulas/physics/high-school/ocjg3wb1esidmka8kwokqqa0x9crrp99wc.png)
where
![[Ar]](https://img.qammunity.org/2020/formulas/physics/high-school/kul7mnccn2ahmbtsujlaxiscbxb4jrisns.png) represents the electronic configuration of argon. This means that all the shells until
represents the electronic configuration of argon. This means that all the shells until
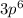 are completed, while the outermost shell:
are completed, while the outermost shell:
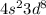
is the one that gives us the number of valence electrons. Since there are 2 electrons in the 4s orbital and 8 electrons in the 3d orbital, the total number of valence electrons is
2 + 8 = 10