Answer:
D) An acid with a small pKa is stronger than an acid with a large pKa
Step-by-step explanation:
Hi, the acity constant (Ka) gives an idea of the strenght of an acid. The higher the Ka, the stronger the acid.
The indicator pKa is obtained by the following formula:
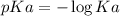
Due to the logarithm, when the Ka increases, the pKa decreases.
Putting all this toghether: An acid with a large Ka has a small pKa and is stronger than an acid with a small Ka (large pKa).
So the correct statement is D