Answer: Thus percentage purity of sodium carbonate is 60.83%.
Step-by-step explanation:
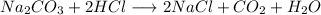
Volume of the HCl solution ,V = 15.55 mL= 0.01555 L
Concentration of HCl solution ,C= 0.1755 M
Moles of HCl in 0.1755 M solution = n
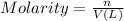
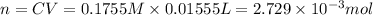
According o reaction 2 moles of HCl reacts with 1 mole of sodium carbonate.
Then n moles of HCl will react with:
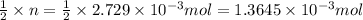 of sodium carbonate.
of sodium carbonate.
Mass of
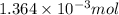 of sodium carbonate:
of sodium carbonate:
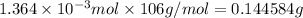
Mass of the sample given= 0.2377 grams
Mass used up in reaction = 0.144585 g
percentage purity=
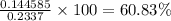
Thus percentage purity of sodium carbonate is 60.83%.