Answer: D. the ionic compound calcium fluoride
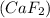
The correct option here is the fourth one.
Step-by-step explanation:
Salt like compounds addition to water is likely to be inducing certain changes in temperature of freezing into solid and also alter the temperature of boiling.
It is noticed that addition of salts in water mostly tends to get lower the freezing temperatures from the original freezing point of water and among the given 3 options calcium fluoride tends to lower the freezing point most when compared to others.
Thus as vant hoff factor is highest factor for
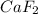 and the freezing point will be lowest.
and the freezing point will be lowest.