Answer:
The correct volume of 42.3% w/w H₂SO₄ is 50,1 mL
Step-by-step explanation:
In order to know the correct volume of 42.3% w/w H₂SO₄ needed, we need to know the mass of H₂SO₄ that is needed. We'll use the wanted concentration, the final volume and the molecular weight of sulphuric acid, thus:
2,7 M * 0,1 L * 98,079 g/mol = 26,48 g of sulphuric acid are needed.
Now we can calculate the correct volume, using the concentration of the available solution, and the density of said solution, thus:
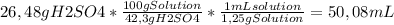
We would then need to measure 50,1 mL (rounded up) of 42.3% w/w H₂SO₄ with the graduated cylinder, and then add it to the volumetric flask.