Answer: Option (d) is the correct answer.
Step-by-step explanation:
According to Bronsted and Lowry, acids are the species which dissociate in water to give hydrogen ions.
Whereas bases are the species which dissociate into water to give hydroxide ions.
Also, like dissolves like. So, when an acid is polar in nature and dissolved in water then it will completely dissociate into ions.
As a result, it will tend to donate hydrogen ions into the solution.
For example,
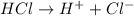
Thus, we can conclude that an acid is a polar substance that dissolves in water and donates hydrogen ions to the solution.