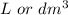Answer:
The Ideal gas law equation is
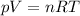
It is the combination of 3 laws
Boyle’s law

Charle’s law
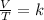
Avagadro’s law
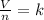
Where k is the constant.
k have been replaced by R and it is called the gas constant.
The value of R depends on the unit of p and V
For example
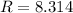 if p is in
if p is in
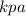 and V in
and V in
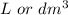
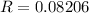 if p is in atm and V in
if p is in atm and V in