Answer:
53.87g
Step-by-step explanation:
Given parameters:
Percentage by mass of Nitrogen in fertilizer = 19%
Mass of fertilizer = 10.0oz
Unkown:
Mass in grams of Nitrogen in the container = ?
Solution
To solve this problem, we first find the percentage of mass of nitrogen in the fertilizer by a taking a percentage of the given mass of the fertlilizer:
Mass of Nitrogen in ounces of the fertilizer =
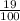 x 10 = 1.9oz
x 10 = 1.9oz
Since we are expected to express this in grams:
1oz = 28.35g
1.9oz = 1.9 x 28.35, 53.87g