The accepted value was either 3.10 g or 3.20 g
Why?
There are two possibilities:
- The accepted value is lower than the experimental value by 1.61%
- The accepted value is higher than the experimental value by 1.61%
The formula for the percent error is the following:
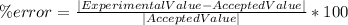
Clearing for Accepted Value and inputting the values we get the following:
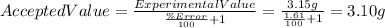
Another possibility is that the the Accepted value is higher. In that case we'd get:
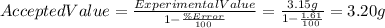 .
.
The most likely value for the accepted value is 3.20 g, as most experiments in which mass is recovered yield a lower experimental value than the initial one.
Have a nice day!