Answer:
71.8 g
Step-by-step explanation:
With the first sentence we can calculate the density of the sample of glycerol:
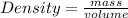
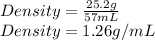
Using the density of the sample we calculate the mass of a given volume:
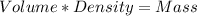
57 mL * 1.26 g/mL = 71.8 g
A 57 mL sample of glycerol weighs 71.8 g.