Answer:
1,076.09 milliliters of olive oil
Explanation:
Remember that
Oil density is 0.92 g/mL
Gasoline density is 0.66 g/mL
1 L=1,000 mL
we know that
The density is equal to the ratio of the mass by the volume
Let
D -----> density in g/mL
m ----> the mass in grams
V ----> the volume in milliliters
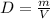
step 1
Find the mass of 1.50 L of gasoline
we have
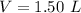
Convert L to mL
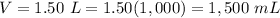
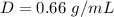
substitute in the formula of density and solve for m
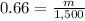
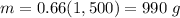
step 2
Find the volume of olive oil that have a mass of 990 grams
so
we have

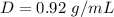
substitute in the formula of density and solve for the volume
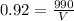
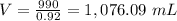
therefore
1,076.09 milliliters of olive oil have the same mass as 1.50 L of gasoline