Answer:
0.541 nm
Step-by-step explanation:
The condition for maxima is,
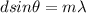
Here, m=0,1,2,.....
And d is the slit separation, m is the order of maxima,
 is the wavelength.
is the wavelength.
Given that, the 17.3 eV electron posses a wavelength of
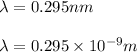
And the order of maxima is
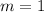 .
.
And the angle at which first order maxima occur is,
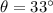 .
.
Put these values in maxima condition while solving for d.
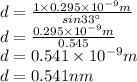
Therefore, the slit separation is 0.541 nm.