Answer:
t = 26541.55 years
Step-by-step explanation:
let the age of charcoal is t
Activity of living material = 15 decays / min /g
Activity of living material = 15 * 1000 decays /min /kg
Acitivity of living material per 0.94 kg A = 0.94 *15 * 1000 decays / min
Activity of after time t is A ' = 5.76*10^2 decay per minute
half life of carbon = 5730 years
designtegration constant
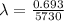
we know
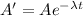
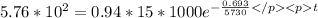
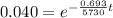
taking log on both side
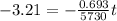
t = 26541.55 years