Answer:
U235 - 968 mg required
Step-by-step explanation:
given data
electric energy Ee = 1910 kWh per month 1910 ×12×3.6×
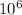 = 8.25 ×
= 8.25 ×
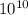 J
J
conversion efficiency = 100%
fission energy Ef = 208 MeV = 3.33 ×
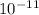 J
J
to find out
How much uranium-235 required
solution
we find no of fission required for U235 that is
no of fission = Ee/ Ef
no of fission =
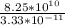
no of fission = 2.48 ×
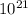
and
mass =
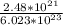 × 235 gm
× 235 gm
we know avogadro no is 6.023 ×
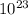
mass = 968 mg
so that 968 mg required