Answer:
Statement 1) False
Statement 2) False
Statement 3) True
Step-by-step explanation:
The uncertainty principle states that " in a physical system certain quantities cannot be measured with random precision no matter whatever the least count of the instrument is" or we can say while measuring simultaneously the position and momentum of a particle the error involved is
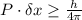
Thus if we measure x component of momentum of a particle with 100% precision we cannot measure it's position 100% accurately as the error will be always there.
Statement 1 is false since measurement of x and y positions has no relation to uncertainty.
Statement 2 is false as both the momentum components can be measured with 100% precision.
Statement 3 is true as as demanded by uncertainty principle since they are along same co-ordinates.