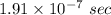Answer:
The time is
Step-by-step explanation:
Given that,
Energy = 2.71 eV
Length = 450 nm
Distance = 2.60 cm
We need to calculate the speed of photon
Using formula of photoelectric emission

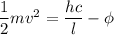
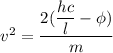
Where, K.E = kinetic energy
h = Planck constant
Put the value into the formula
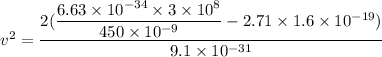
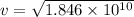
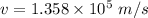
We need to calculate the time
Using formula of distance
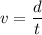
Put the value into the formula
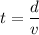
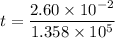
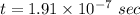
Hence, The time is