Answer:
16 m / s
Step-by-step explanation:
Average kinetic energy of one mole of a gas is given by the expression
K.E = 3 / 2 R T { T is absolute temperature of the gas }
Putting the values , we get
4070 = 3 / 2 X 8.312 X T
T = 326.35 K
Under similar condition temperature of oxygen will also be same . So temperature of oxygen gas will be 326.35 K.
Now ,RMS value of a gas =
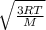
T is absolute temperature of gas and M is molecular mass.
Molecular mass of oxygen is 32 gm . T is 326.35
RMS speed =
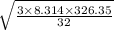
= 16 m / s . approx.