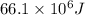Answer:
Amount of necessary heat transfer, q =
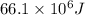
Given:
Volume of liquid Nitrogen, V = 0.200

Density of liquid Nitrogen,
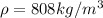
Temperature,
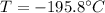
Temperature Rise, T' =
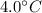
Latent heat of Vaporization,
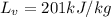
Specific heat capacity of gas,
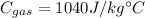
Solution:
Now, calculation of heat transfer required to evaporate liquid nitrogen and raise its temperature is given by:
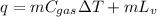 (1)
(1)
Now, mass, m can be given by:
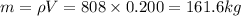
Now, from eqn (1):
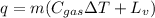
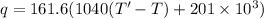
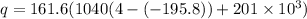
q =