Answer:
mass of U 235 is 8.92 ×
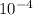 gram
gram
Step-by-step explanation:
given data
electric energy = 1760 kWh per month
energy u 235 = 208 MeV
to find out
mass of u 235
solution
we know here energy consume in a year is
energy consume = 1760 × 10³ ×3600 × 12
energy consume = 7.603 ×
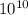 J
J
and
now we find no of u235 atom by burn require energy is
that is = energy consume / energy
= 7.603 ×
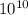 / ( 208 × 1.6 ×
/ ( 208 × 1.6 ×
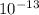 )
)
= 2.285 ×
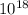
and we know 1 mole of u235 = 6.02 ×
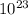 atoms
atoms
so mass of U235 is
mass = 2.285 ×
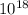 / ( 6.02 ×
/ ( 6.02 ×
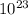 )
)
mass of U 235 = 8.92 ×
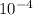 gram
gram