Answer:
P = 2.91*10^{-24} kg m/s

size of atom hat lie in range of 1 to 5 Angstrom
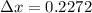 Angstrom
Angstrom
Step-by-step explanation:
A) MOMENTUM
p = mv
where m is mass of electron
so momentum p can be calculated as
p = 9.11*10^{-31} *3.2*10^{6}
P = 2.91*10^{-24} kg m/s
b) wavelength
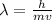
where h is plank constant
so
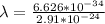

c) size of atom hat lie in range of 1 to 5 Angstrom
d) from the information given in the question we have
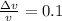
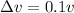
we know that
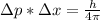
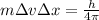
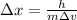
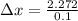 [
[
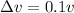 ]
]
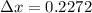 Angstrom
Angstrom