Answer:
4.63 p.m.
Step-by-step explanation:
The problem given here can be solved by the Compton effect which is expressed as
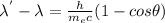
here,
 is the initial photon wavelength,
is the initial photon wavelength,
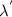 is the scattered photon wavelength, h is he Planck's constant,
is the scattered photon wavelength, h is he Planck's constant,
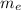 is the free electron mass, c is the velocity of light,
is the free electron mass, c is the velocity of light,
 is the angle of scattering.
is the angle of scattering.
Given that, the scattering angle is,
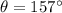
Putting the respective values, we get
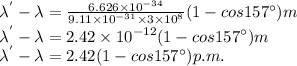
Therfore,
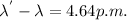
Here, the photon's incident wavelength is
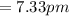
So,
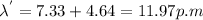
From the conservation of momentum,
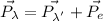
here,
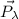 is the initial photon momentum,
is the initial photon momentum,
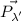 is the final photon momentum and
is the final photon momentum and
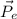 is the scattered electron momentum.
is the scattered electron momentum.
Expanding the vector sum, we get
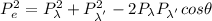
Now expressing the momentum in terms of De-Broglie wavelength
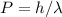 and putting it in the above equation we get,
and putting it in the above equation we get,
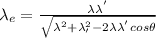
Therfore,
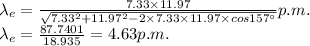
This is the de Broglie wavelength of the electron after scattering.