Answer:
The correct option is 'c':electron,proton,helium nucleus
Step-by-step explanation:
The De-Broglie's wavelength of particle is given by
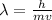
Thus we can see that wavelength is inversely related to mass of the particle since 'h' (Plank's constant) and velocity is same for all the particles
Thus we conclude that the the lightest particle will have the most wavelength
Electron being the lightest of the 3 particles will have the largest wavelength thus the correct option is 'c'. Since electron has the largest wavelength followed by proton and the least wavelength among the 3 is of helium.