Answer: Option (C) is the correct answer.
Step-by-step explanation:
It is known that a weak acid upon dissociation will lead to the formation of a strong conjugate base. Whereas if an acid is strong then it will lead to the formation of a weak conjugate base.
For example, HF is a weak acid and when it will dissociate then it will give hydrogen ion and fluroide ion.
The reaction will be as follows.
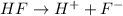
Here,
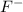 is the conjugate base and
is the conjugate base and
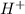 is the conjugate acid.
is the conjugate acid.
Whereas HCl is a strong acid and its
 will be a weak conjugate base. Similarly,
will be a weak conjugate base. Similarly,
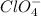 and
and
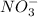 are also weak base.
are also weak base.
Thus, we can conclude that out of the given options
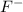 is the strongest base.
is the strongest base.