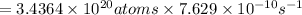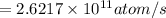Answer:
Activity of Sr isotope is
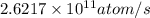 .
.
Step-by-step explanation:
Mass of the Sr isotope = 50 mg =0.050 g
Moles of Sr isotope =
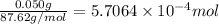
Number atoms of of Sr isotope:
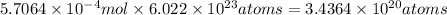
Half life of the Sr =
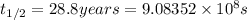
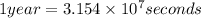
Activity of the Sr isotope = k
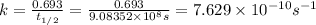
Activity in atom/second:
= Number of atoms × k