Answer:The change in internal energy ,
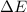 is 8.09 kJ
is 8.09 kJ
Explanation:
According to first law of thermodynamics:
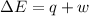
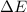 =Change in internal energy
=Change in internal energy
q = heat absorbed or released = +12.43 kJ (heat absorbed is positivew = work done or by the system = - 4.34 kJ (Work done by the system is negative)
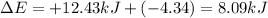
Thus change in internal energy is 8.09 kJ