Answer:
E = 3.74 X 10^{-7} N/C
upward direction
Step-by-step explanation:
the force from an electric field is F = qE
as in the given question, three electron is missing in the ion, so, it has a positive charge of-
(3)(1.6 X 10-19) = 4.8 X 10^{-19} C
To levitate, the electric force must match the weight of the ion
so qE = mg
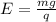
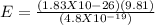
E = 3.74 X 10^{-7} N/C
Since the charge is positive therefore the Field will point in upwards direction.