Answer: The amount of heat released by KCl is 148.6 J and the reaction is endothermic in nature.
Step-by-step explanation:
Exothermic reactions are defined as the reaction in which heat is released.
Endothermic reactions are defined as the reaction in which heat is absorbed.
As, temperature of water is decreasing, this means that water is absorbing the heat.
So, amount of heat released by KCl will be equal to the amount of heat absorbed by water.
To calculate the change in temperature, we use the equation:
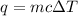
where,
q = heat absorbed = ?
m = mass of water = 35.2 g
c = specific heat capacity of water = 4.18 J/g.°C
 = 1.01 °C
= 1.01 °C
Putting values in above equation, we get:
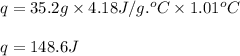
Hence, the amount of heat released by KCl is 148.6 J and the reaction is endothermic in nature.