Answer:
no. of water molecules associated to each molecule of
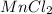 = 4
= 4
Step-by-step explanation:
Mass of
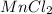 before heating = 19.8 g
before heating = 19.8 g
Mass of
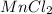 after heating = 12.6 g
after heating = 12.6 g
Difference in mass of
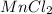 before and after heating
before and after heating
= 19.8 - 12.6 = 7.2 g
Difference in mass corresponds to mass of water driven out.
Molar mass of water = 18 g/mol
No. of moles of water =
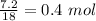
Mass of
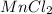 obtained after heating is mass of anhydrous
obtained after heating is mass of anhydrous
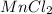 .
.
Mass of anhydrous
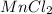 = 12.6 g
= 12.6 g
Molar mass of
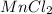 = 125.9 g/mol
= 125.9 g/mol
No. of mol of anhydrous
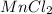 =
=

so,
0.1 mol of
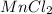 have 0.4 mol of water
have 0.4 mol of water
1 mol of
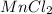 will have =
will have =
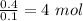
Hence, no. of water molecules associated to each molecule of
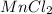 = 4
= 4