Answer: The molarity of bromide ions is 0.348 M.
Step-by-step explanation:
To calculate the moles of cadmium nitrate, we use the equation:
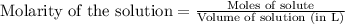 .....(1)
.....(1)
Molarity of silver nitrate = 0.7170 M
Volume of silver nitrate = 24.52 mL = 0.02452 L (Conversion factor: 1 L = 1000 mL)
Putting values in equation 1, we get:
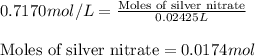
The chemical equation for the reaction of silver nitrate and bromide ions follows:
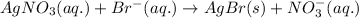
By Stoichiometry of the reaction:
1 mole of silver nitrate reacts with 1 mole of bromide ions.
So, 0.0174 moles of silver nitrate will react with =
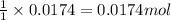 of bromide ions.
of bromide ions.
Now, calculating the molarity of bromide ions by using equation 1, we get:
Moles of bromide ions = 0.0174 moles
Volume of solution = 50 mL = 0.05 L
Putting values in equation 1, we get:
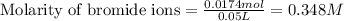
Hence, the molarity of bromide ions is 0.348 M.