Answer:
D) Heat capacity
Step-by-step explanation:
Entropy is defined as
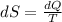
Where
dS: is an infinitesimal amount of entropy
dQ: is an infinitesimal amount of heat
T: is the temperature in absolute units
The units of this are units of heat over units of temperature.
This is the same unit used by heat capacity
![[(J)/(K)]](https://img.qammunity.org/2020/formulas/engineering/college/pjt1xcwmc3y1wqraaziu4o2aphly7hfei4.png)
Do not confuse it with the specific heat capacity. Heat capacity is a property of a body and is an extensive property, while specific heat capacity (sometimes shortened to specific heat) is a property of material and is an intensive property.