Answer:
P = 0.44 atm
Step-by-step explanation:
given data:
gas constant R = 0.0821 L.atm/mol. k
Temperature = 19 degree celcius = 19+273 = 292 K
Volume = 40.5 L
FROM IDEAL GAS EQUATION IS WE HAVE
mole n = 0.75
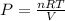
putting all value to get the desired value to get desired pressure value.
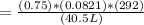
P = 0.44 atm