Answer:
21.28 kg
Step-by-step explanation:
Density of iron = 7890 kg/m³ =
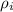
Mass of iron = 8.7 kg=
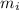
Volume of gold = Volume of iron = v
Density = Mass / Volume
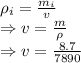
Density of gold = 19300 kg/m³=
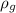
Mass of iron =
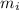
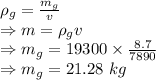
Mass of gold needed to make the new model is 21.28 kg