Answer:
Oxygen atom, because the partial negative charge is localized on the oxygen atom.
Step-by-step explanation:
A cation is a positive ion, so to associate with it, would be necessary a negative ion, an anion. When the water dissociates, it will form
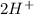 and
and
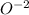 .
.
So, oxygen has a negative charge, and in the molecule, the partial negative charge because it's more electronegative. Then, oxygen will be associated with the cation.